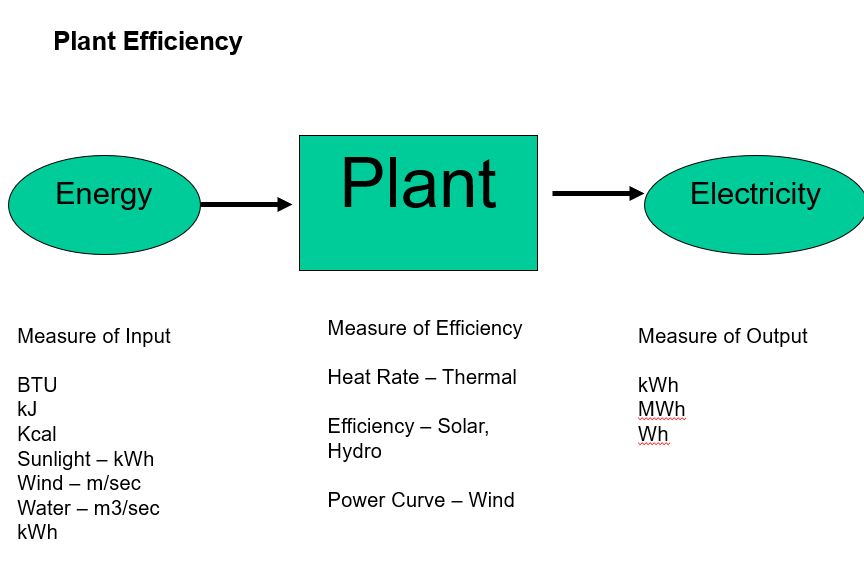
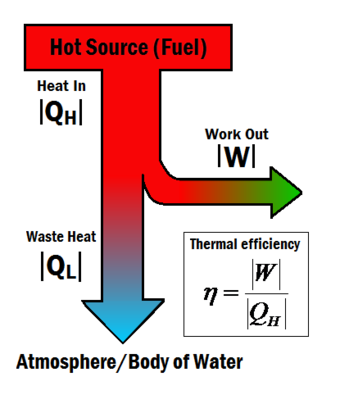
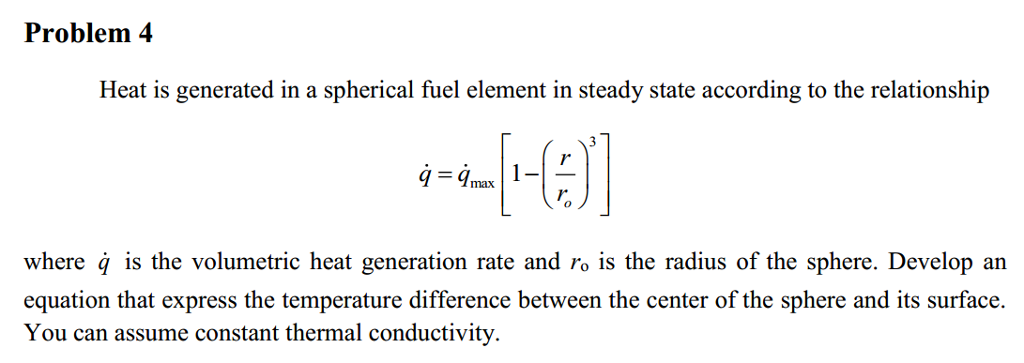
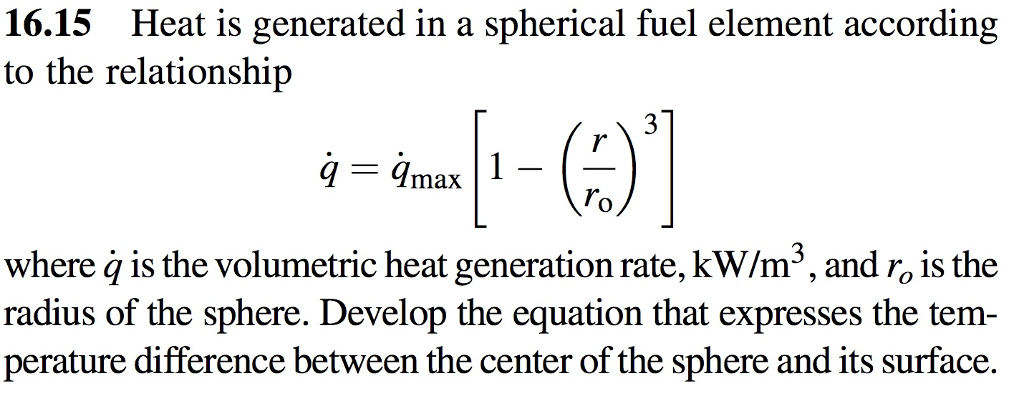OneClass: 6) Global warming by CO2, emissions in the burning of fossil fuels is an environmental conc...

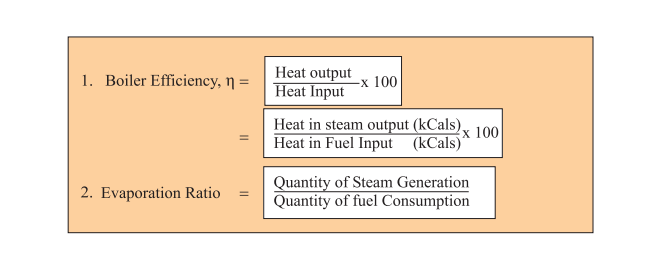
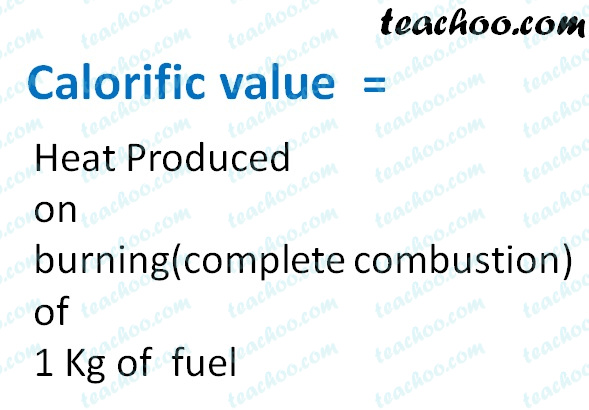
In an experiment 4.5 kg of a fuel was completely burnt. The heat produced was measured to be 1,80,000 kJ. Calculate the calorific value of the fuel(in kJ/kg)?
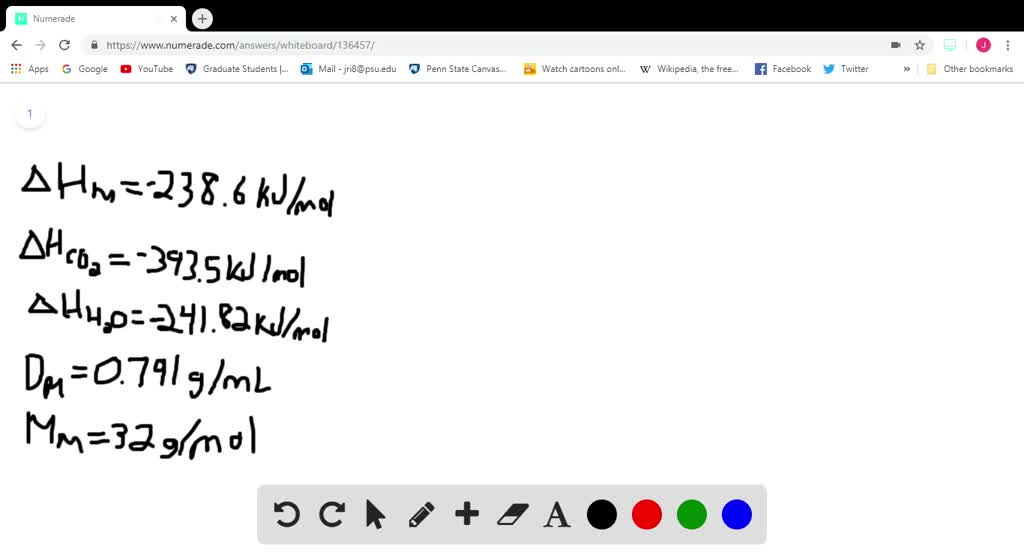
SOLVED:Methanol (CH3 OH) is used as a fuel in race cars. (a) Write a balanced equation for the combustion of liquid methanol in air. (b) Calculate the standard enthalpy change for the

In an experiment, 4.5 kg of a fuel was completely burnt. The heat produced was measured to be 180,00
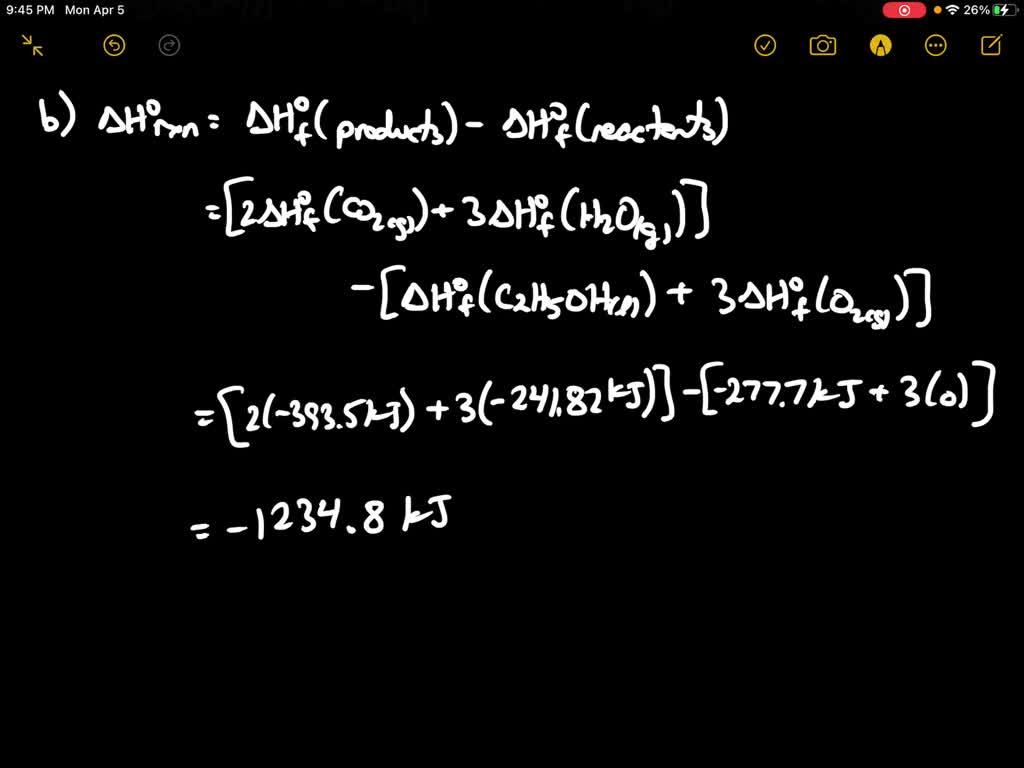
SOLVED:Ethanol gas (C2 H6 O) is burned with 110 percent theoretical air. During the combustion process, 90 percent of the carbon in the fuel is converted to CO2 and 10 percent is