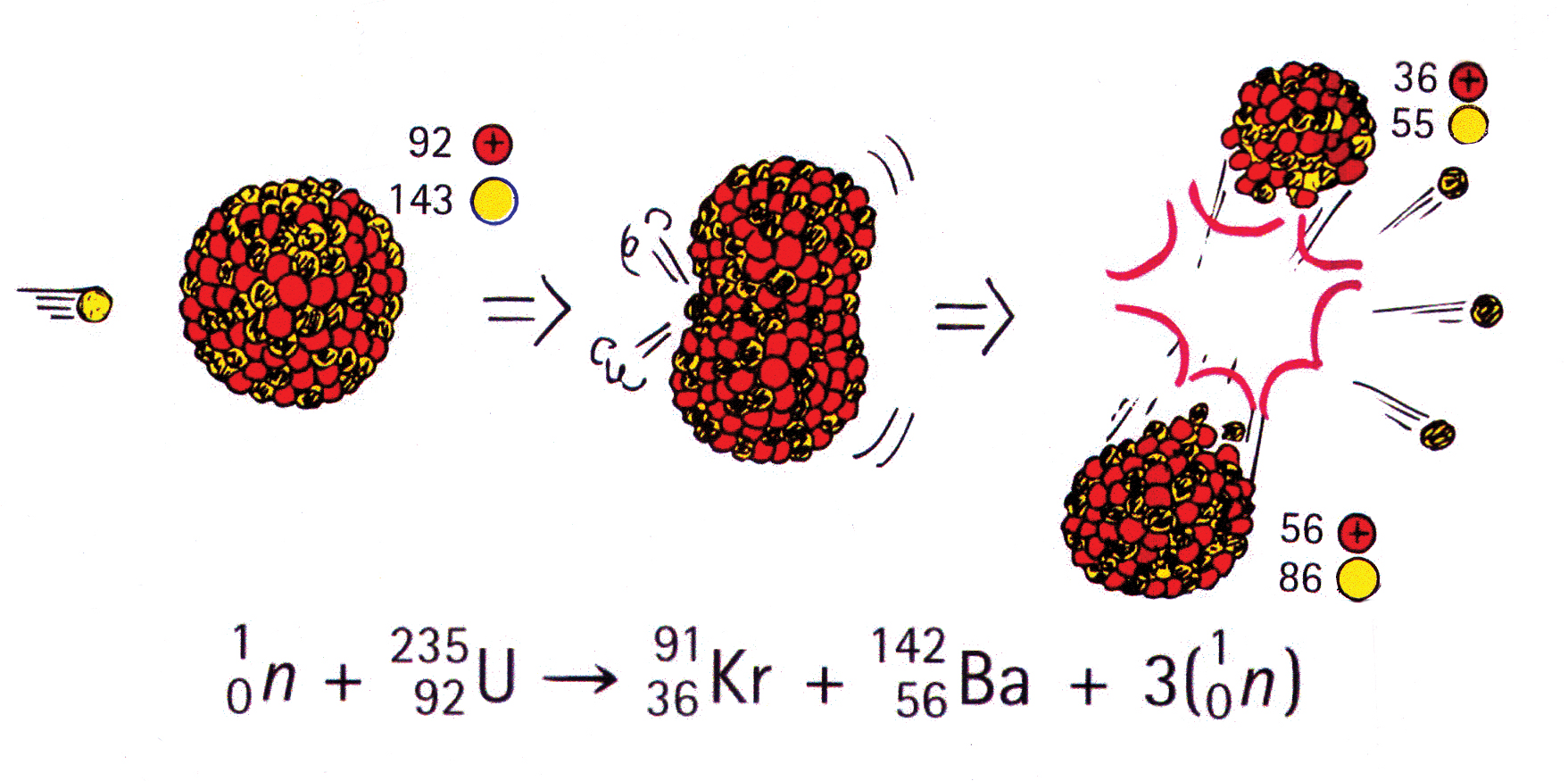
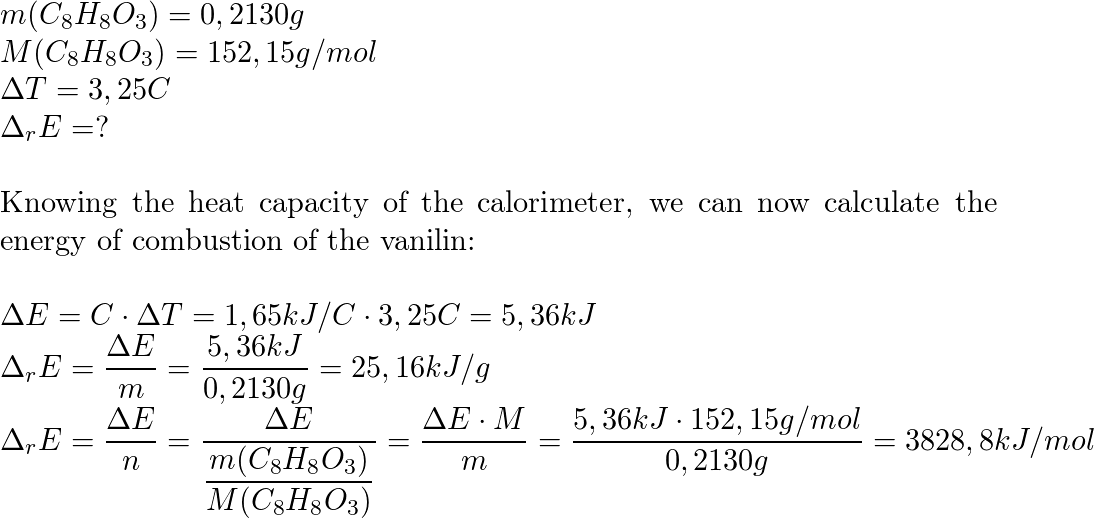An explosion of atomic bomb releases an energy of 7.6xx10^(13)J. If 200 MeV energy is released on fission of one .^(235)U atom calculate (i) the number of uranium atoms undergoing fission. (ii)

For complete combustion of ethanol, C2H5OH(l) + 3O2(g)→ 2CO2(g) + 3H2O(l) , the amount of heat produced as measured in bomb calorimeter is 1364.47 kJ/mol at 25^C . Assuming ideally, the enthalpy
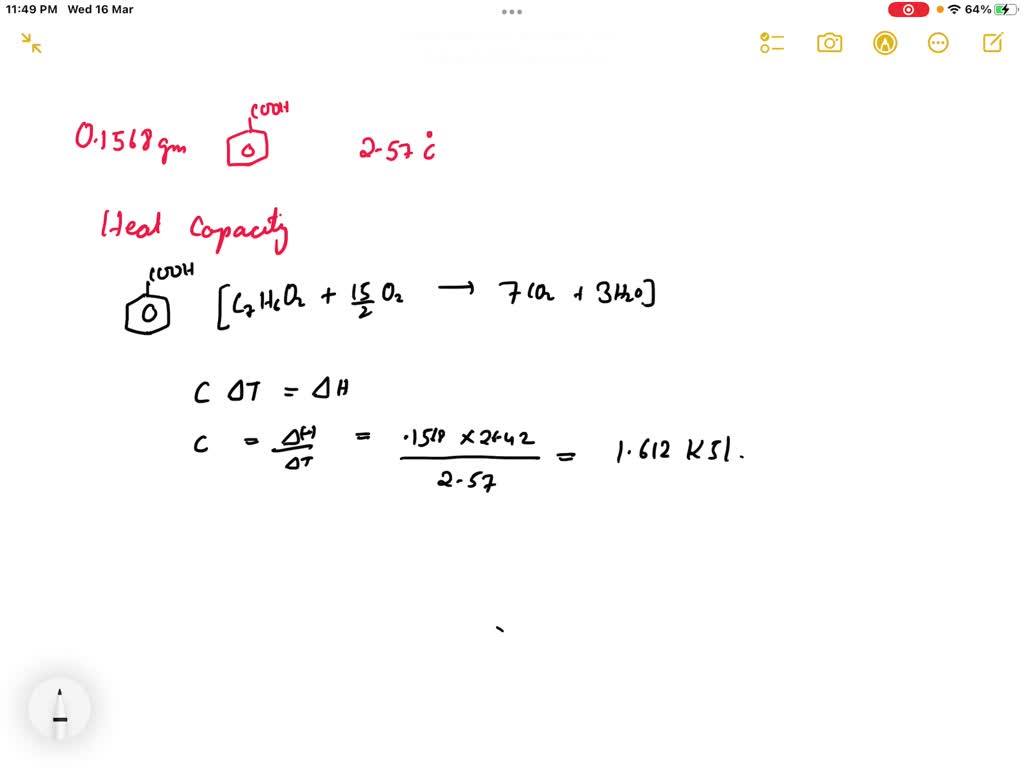
SOLVED: The combustion of 0.1568 g benzoic acid increases the temperature of a bomb calorimeter by 2.57°C. Calculate the heat capacity of this calorimeter. (The energy released by combustion of benzoic acid

An explosion of atomic bomb releases an energy of 7.6xx10^(13)J. If 200 MeV energy is released on fission of one .^(235)U atom calculate (i) the number of uranium atoms undergoing fission. (ii)
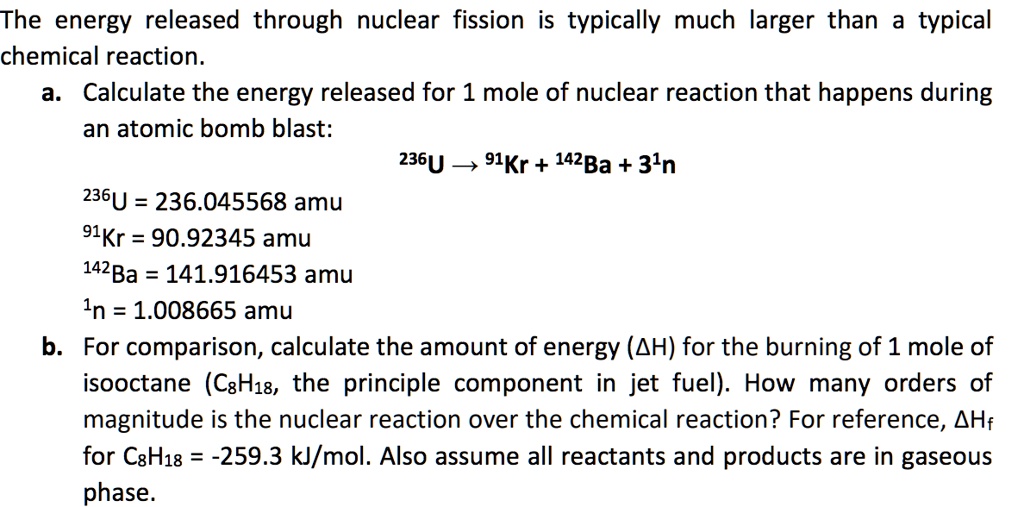
SOLVED: The energy released through nuclear fission is typically much larger than typical chemical reaction Calculate the energy released for 1 mole of nuclear reaction that happens during an atomic bomb blast: